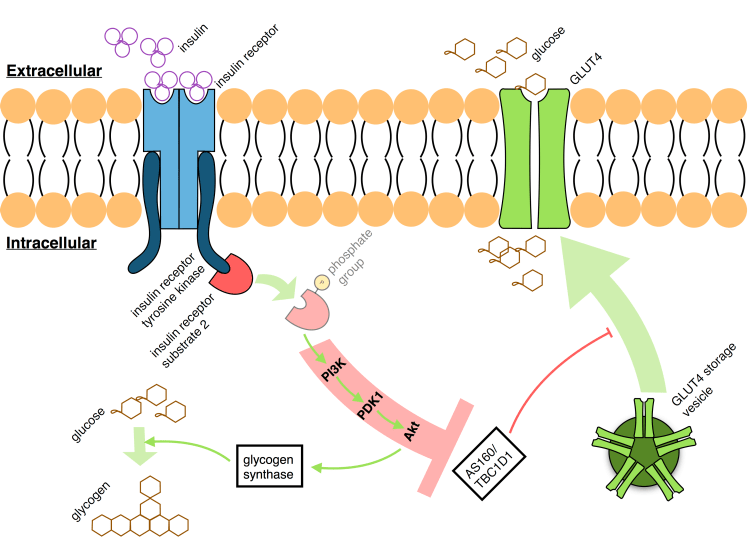
The role of glucose in the body is primarily to maintain glucose homeostasis by signalling the removal of excess glucose from the blood stream. This is achieved through the conversion of glucose to glycogen in the muscular and hepatic tissues. This is the process through which glucose is removed from the blood plasma posterior to feeding and requires the activation of glycogen synthase in response to raised insulin levels. For instance, type I diabetes patients synthesise lower quantities of insulin and consequently synthesise only one third the glycogen of healthy adults.
This process is mediated through two primary pathways: Firstly, the presence of glucose inhibits the glycogen phosphorylase enzyme and prevents the breakdown of existing glycogen stores. Secondly, the pulsatile delivery of pancreatic insulin in response to glucose will bind the insulin receptors on target cells and activate the intracellular domain, insulin receptor tyrosine kinase (IRTK). Activated IRTK is then capable of phosphorylating the insulin receptor substrate (IRS)-2, itself a major docking protein for the phosphatidylinositol 3-kinase (PI3K). Beyond this, PI3K is well known to activate Akt through 3’-phosphoinositide dependent kinase 1 (PDK1). Glucose is then disposed of through the Akt-mediated phosphorylation, and activation, of glycogen synthase via the glycogen synthase kinase. This body-wide feedback system allows for the body’s glucose intake to be initially converted into energy before an endocrine response which begins to store this energy and limit the immediate exposure to hyperglycæmia.
Another step in the process allows the body and individual cells to limit or upregulate their glucose uptake in response to incoming insulin. In order for glucose to enter the cell, it must cross the membrane via a transmembrane glucose transporter (GLUT). In insulin-mediated GLUT4 translocation to the cellular membrane, GLUT4 transporters are collected within GLUT4 storage vesicle (GSV) complexes and conveyed to the membrane by specialised Rab proteins. To achieve this, insulin-activated Akt will in turn activate Rab GTPases through either Akt substrate of 160 kDa (AS160), TBC1 domain family member 1 (TBC1D1), or likely both. Inability to synthesise or produce either of these proteins produced an excessive extracellular glucose concentration and chronic glucose intolerance within mice. Recalling that insulin indirectly phosphorylates Akt, the insulin receptor may therefore control both glycogen synthesis, glucose synthesis from glycogen, and transmembrane glucose transport.
Much evidence suggests, however, that the Akt pathway is not the only pathway involved in glycogenesis mediated through insulin. Deleting Akt1 and Akt2, for example, did not result in the depletion of glycogen stores within the cell. Increasing insulin concentrations in a homeostatic glucose environment insignificantly increased Akt but led to significant decreases in glucose production. On the other hand, blocking Akt and Rac1 simultaneously, however, completely prevents the uptake of glucose to the cell, where both of their pathways have been implicated specifically in GLUT4 transport to the cell membrane. Therefore, glucose is removed from the blood via GLUT4 and subsequently converted in glycogen before storage, whilst insulin-mediates this process via Akt and Rac1 and through upregulation of glycogen synthesis and GLUT4 translocation to the membrane.
(The prior instalment of this series of posts is entitled “Reviewing Diabetes: 0. A basis for understanding”, whilst “Reviewing Diabetes: 2. Insulin resistance” is to follow.)